
|
8. Biophysical characterization of lipids and lipid/protein interactions |
|
Cells are surrounded by a biological membrane build of lipids and proteins that protect the cell from its surrounding. And keep the internal components of the cell from diffusing away. This biological membrane consists of a matrix of lipid molecules, which forms a bilayer. Proteins either span the membrane (integral membrane proteins) or are attached to it via interactions with another protein, a lipid anchored covalently to the protein, or via combinations of the above (membrane associated proteins). Many membrane associated proteins contain special amino acid sequences that fold into a specific docking module. The specific interaction of membrane associated proteins with lipids can be accomplished in a number of ways, but mainly by means of electrostatic and hydrophobic interactions accompanied by the formation of hydrogen bonds between the protein and lipid. The folding of the binding domain to “fit” a specific lipid is a commonly encountered theme. In particular, we are interested in what sets certain lipids apart as a protein binding partner and conversely why a specific protein binds to a specific lipid or lipids. In order to understand the mechanism of binding we therefore characterize both the phase behavior and electrostatic properties of lipids. Our research is described in more detail in the following sections.
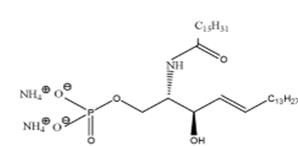
Ceramide-1-phosphate Ceramide-1-phosphate (Fig. 1), is a minor but important sphingolipid. It is involved in a number of diverse intracellular processes, such as cell differentiation and the inflammatory response. Using x-ray diffraction, nuclear magnetic resonance and differential scanning calorimetry we are currently involved in characterizing the phase behavior and electrostatic properties of this important sphingolipid.
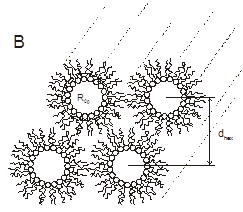
Spontaneous radius of curvature The spontaneous radius of curvature is a description of the molecular shape of a lipid. It describes its propensity to form non-bilayer phases. The spontaneous curvature of any (small) membrane component can be determined using a protocol developed by Rand et al. We are particularly interested in the effect of acylchain composition on the spontaneous curvature of lipids and on the potential role of viral fusion peptides on the spontaneous curvature of the membrane.
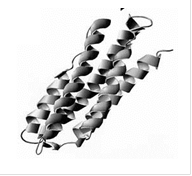
Lipids are also important dietary metabolites. As such these hydrophobic lipids (such as triacyl glycerols, cholesterol esters, etc.) need to be packaged in specialized containers, namely lipoprotein particles (for trafficking of fat in our bloodstream) and in adipocites (for the storage of fat inside our cells). One type of specialized protein involved in this packaging is called the exchangeable apolipoprotein or exchangeable lipid droplet protein. It is called exchangeable since it is able to traffic between different particles and between the particle and the cytosol/blood. However, how these proteins accomplish this is only partly understood. We are currently using a diverse array of biophysical techniques in order to fully characterize these interactions. As a model apolipoprotein we currently focus our efforts on the exchangeable apolipoprotein, apoLp-III from the grasshopper locusta migratoria.
Lipid electrostatics Amino acid residues in proteins come in many different flavors, and in particularly can be both positively and negatively charged. Lipids are either neutral, zwitterionic (carry both a positive or negative charge) or exclusively negatively charged with the rare exception of sphingosine(phosphate) which contains a free amine group that depending on pH can carry a positive charge. The negatively charged membrane lipids are ideally suited for electrostatic interaction with positively charged protein membrane domains. Using magic angle spinning 31P-NMR we study the ionization properties of the negatively charged membrane lipids. In particular, we are interested in the roles of different membrane components on the pKa values of negatively charged lipids. Furthermore using 31P MAS NMR we probe the interaction of different peptides that have a specific affinity for an anionic lipid, thereby trying to understand the molecular (electrostatic) basis of the interaction. |
|
Figure 1. Chemical structure of C16-ceramide-1- phosphate |
|
Figure 2. Cross sectional view of the inverted hexagonal phase (HII). |
|
Figure 4. Three dimensional ribbon diagram of apoLp-III from locusta migratoria |
|
Publications and Presentations Edgar E. Kooijman, Jesús Sot, L.-Ruth Montes, Alicia Alonso, Arne Gericke, Ben de Kruijff, Satyendra Kumar, and Felix M. Gońi, Membrane organization and ionization behavior of the minor but crucial lipid ceramide-1-phosphate, Biophysical Journal 94:4320-4330 (2008).
Poster presentations at the 52nd annual meeting of the Biophysical Society, Long Beach, February 2008 1. Structure of cer-1-p at the air-water interface in the absence and presence of Ca2+. 2. Membrane organization and ionization behavior of minor but crucial lipid ceramide-1-phosphate.
Poster presentation at the 51st annual meeting of the Biophysical Society, Baltimore, March 2007 |
